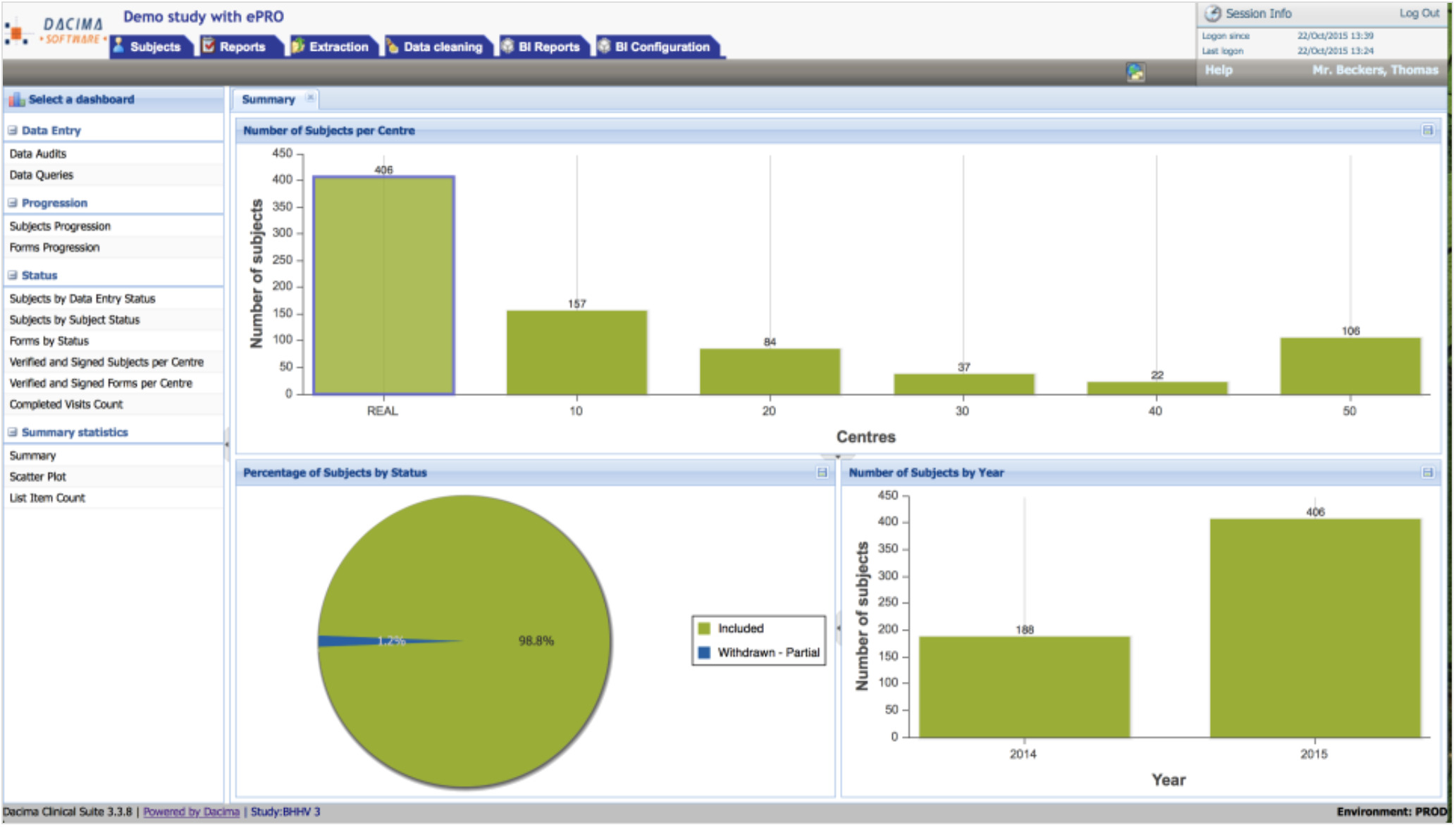
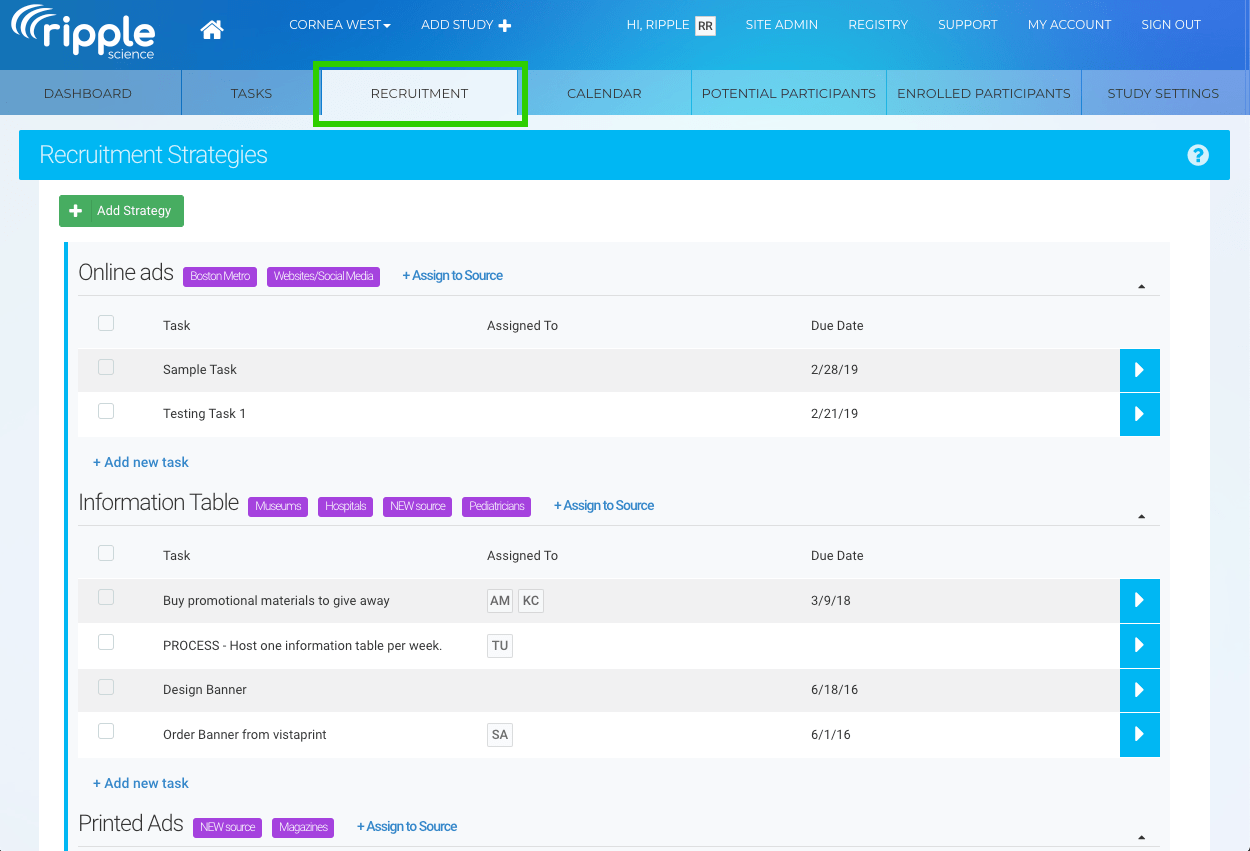
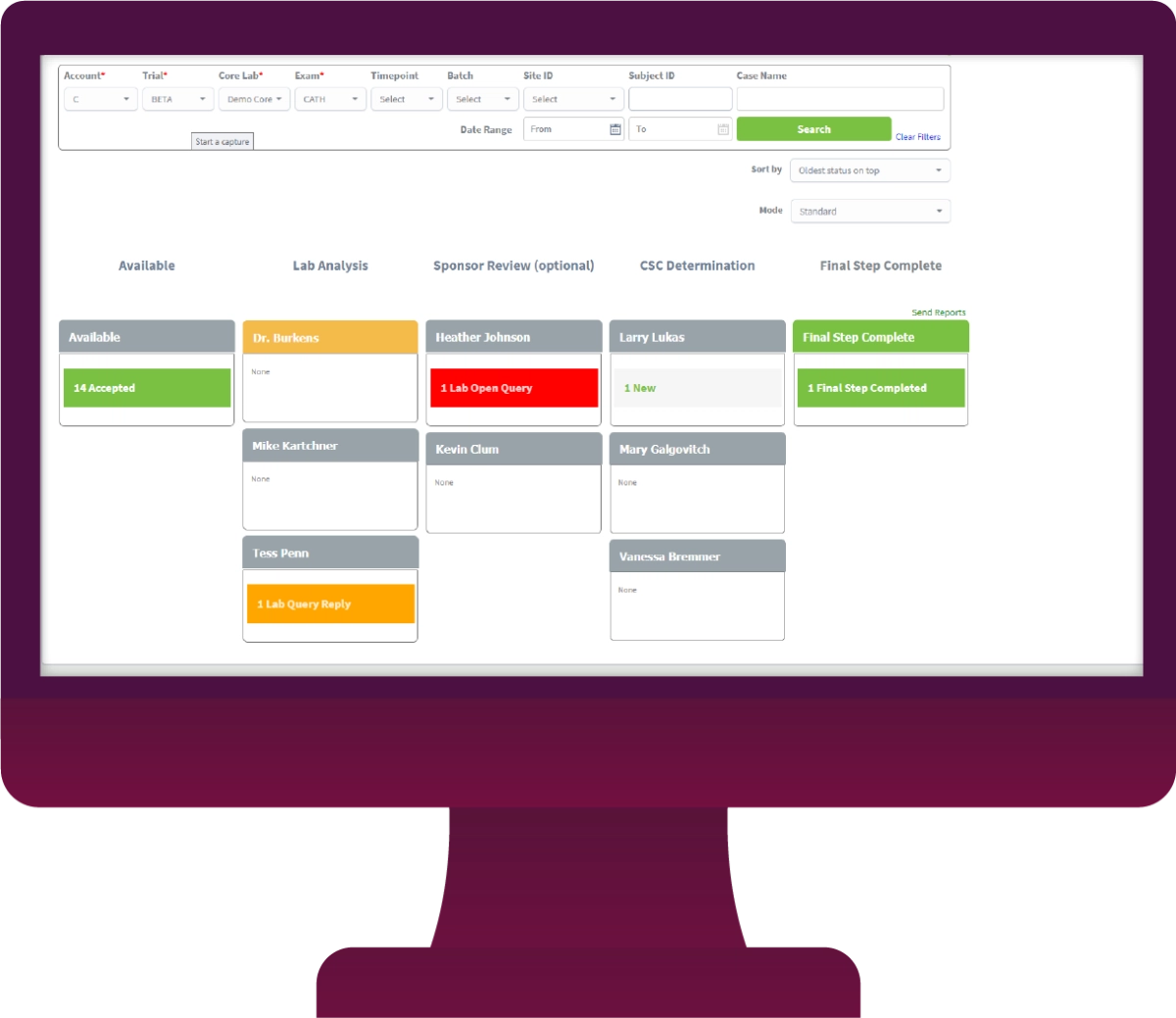
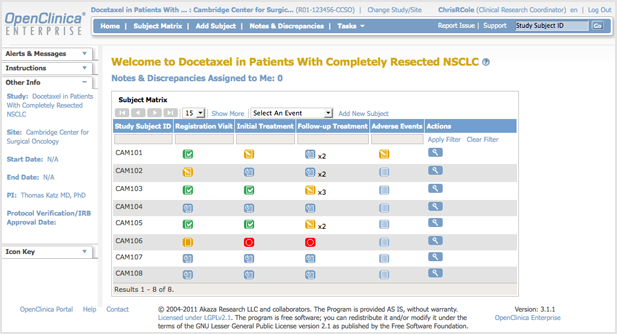
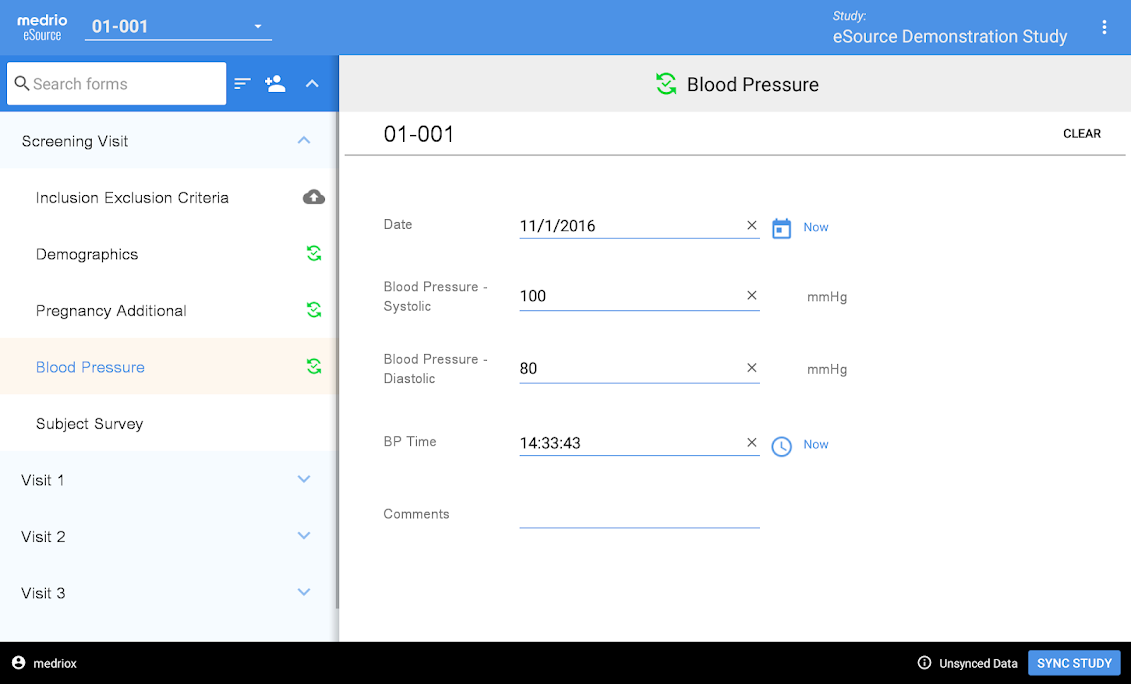
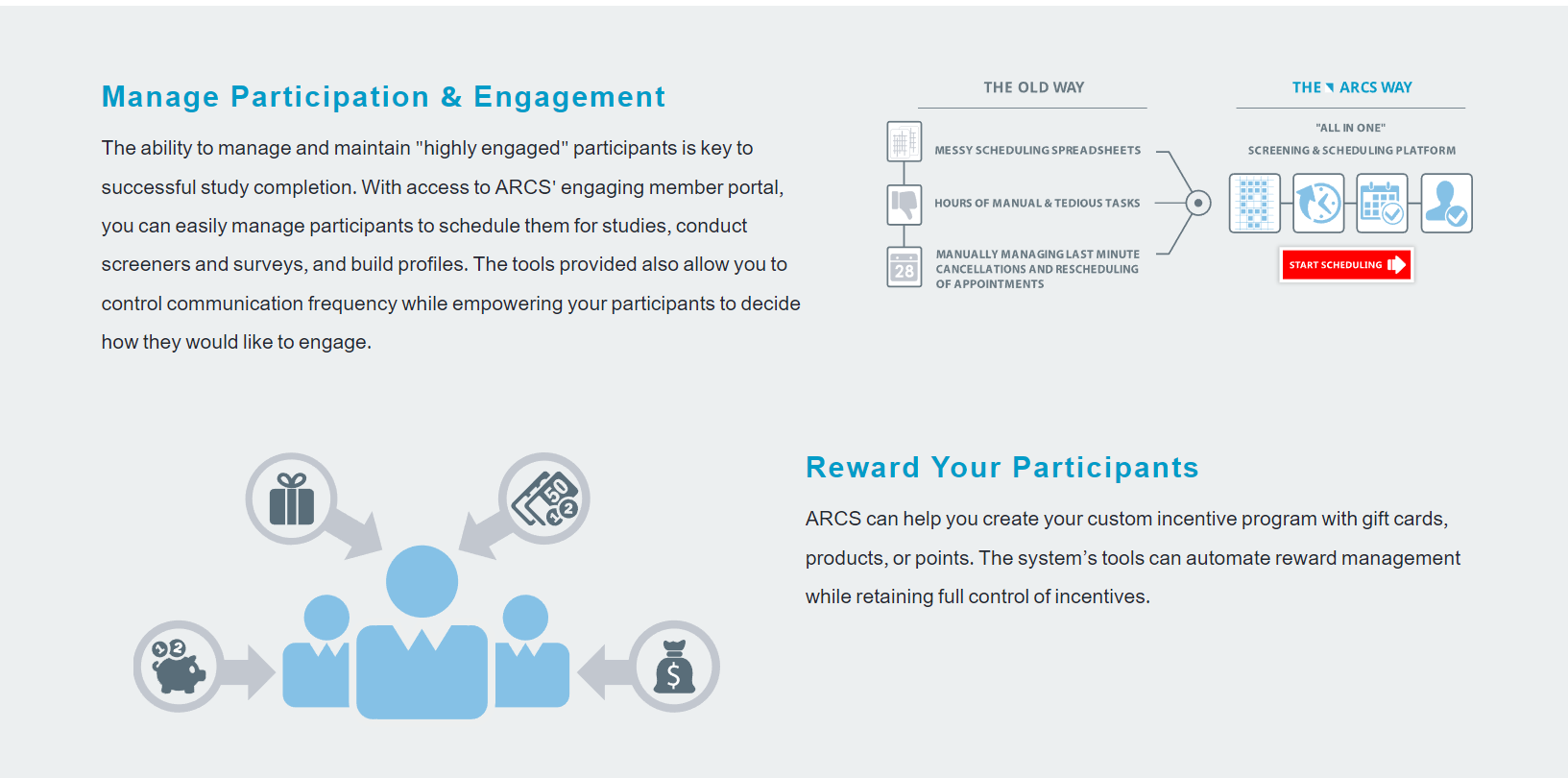
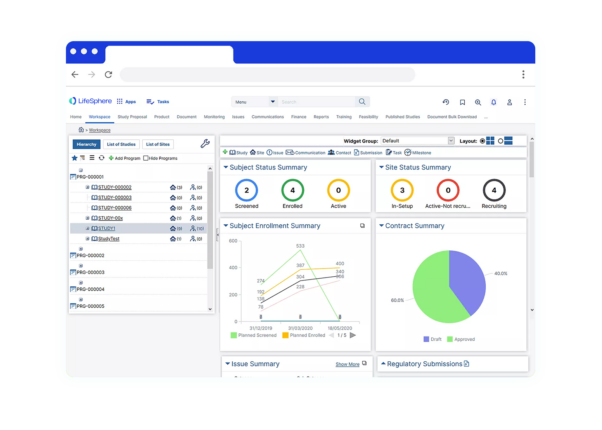
Clinical Conductor is an all-inclusive CTMS that can be scaled to meet the needs of any research site, site network, hospital, or health system.
You can use the instrument to boost patient enrollment and retention rates. It helps you streamline the process of recruiting patients, participants, and staff so that you'll be able to do more in less time. Sharing and viewing documents is a breeze, as is communicating with colleagues and external partners. You can also avoid the hassle and security issues associated with paper papers by having your clients sign electronically.
There are a bunch of decent tools out there that offer the same array of services as Clinical Conductor CTMS. And it can sure get confusing to choose the best from the lot. Luckily, we've got you covered with our curated lists of alternative tools to suit your unique work needs, complete with features and pricing.