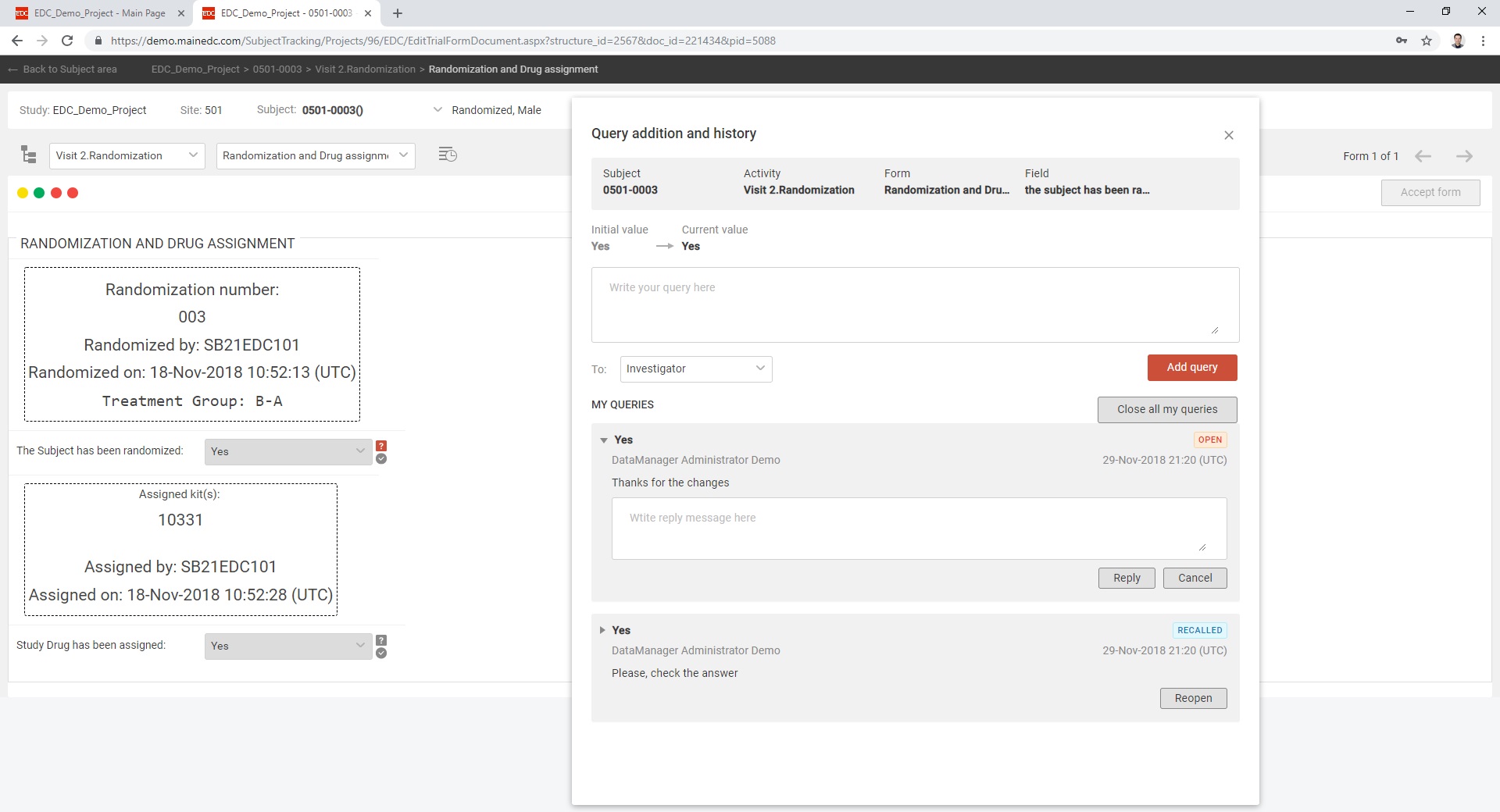
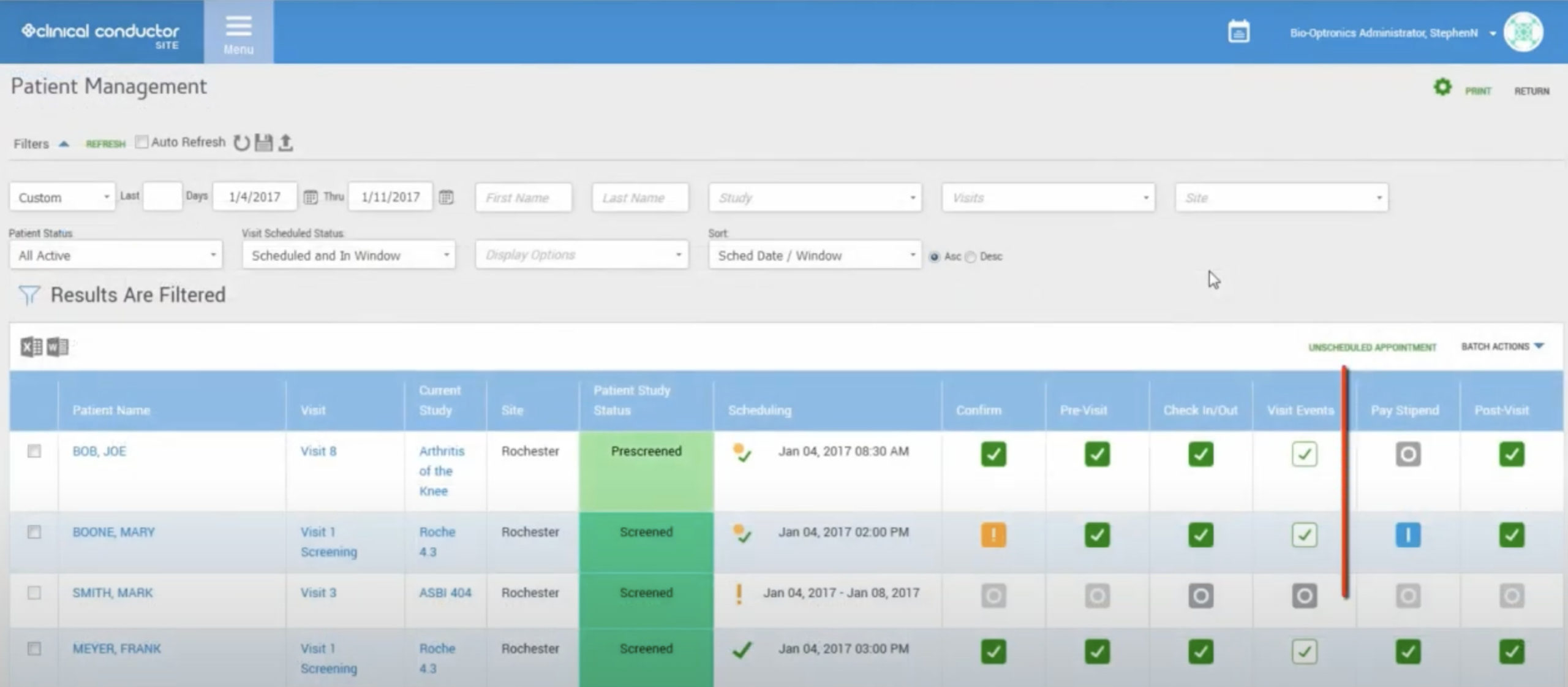
LifeSphere CTMS's capacity to streamline the clinical trial design, management, and closure procedures is useful for research institutes, and pharmaceutical enterprises of all sizes.
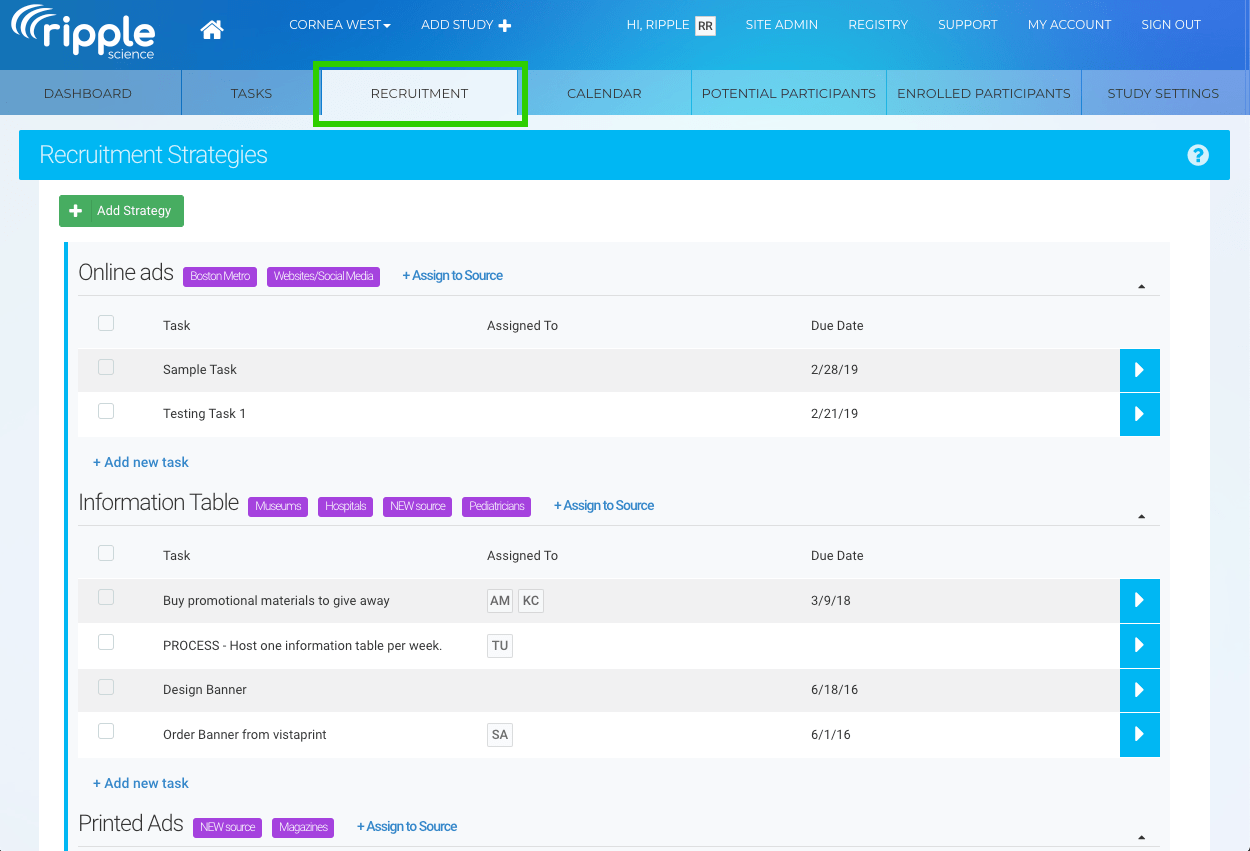
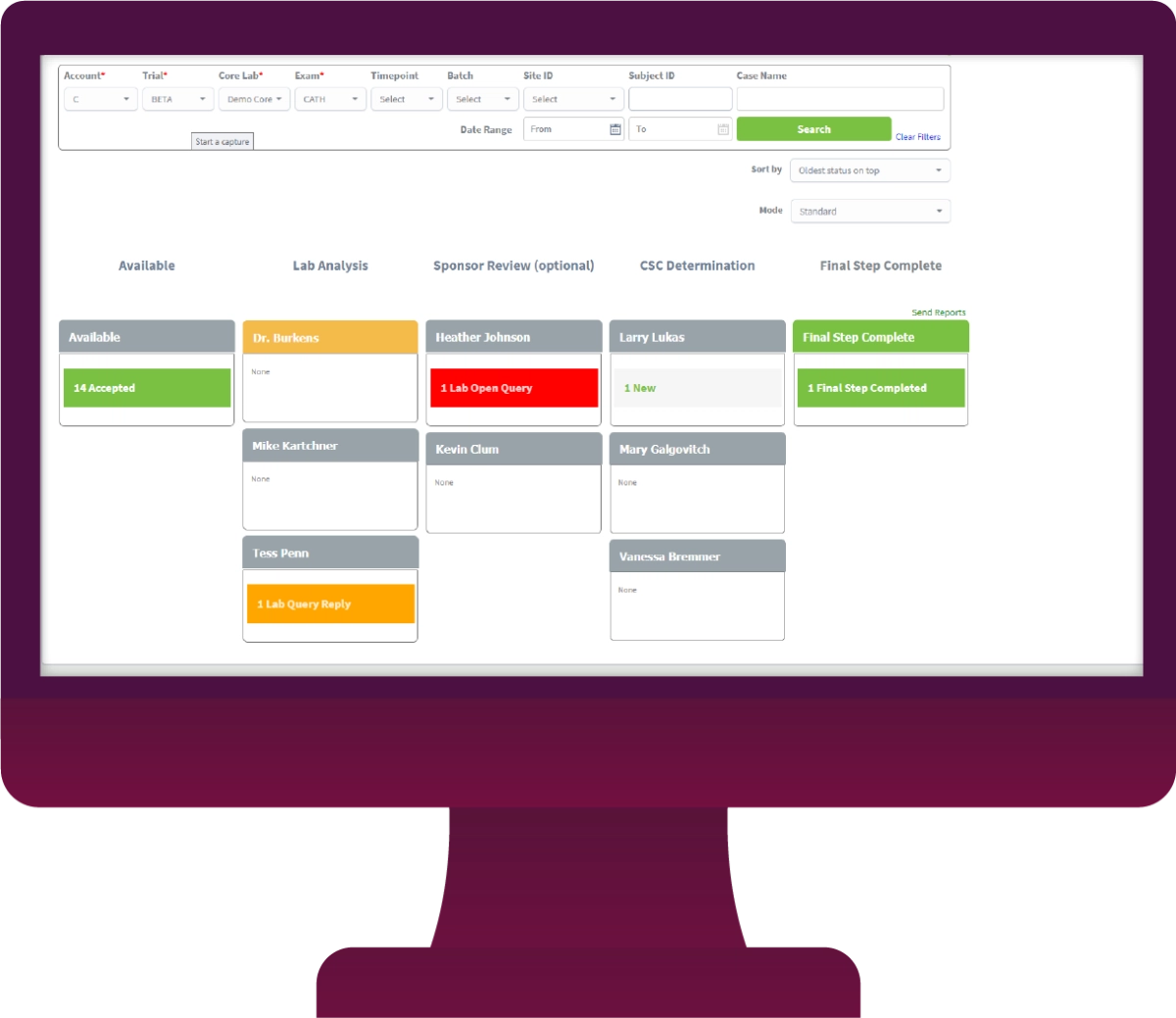
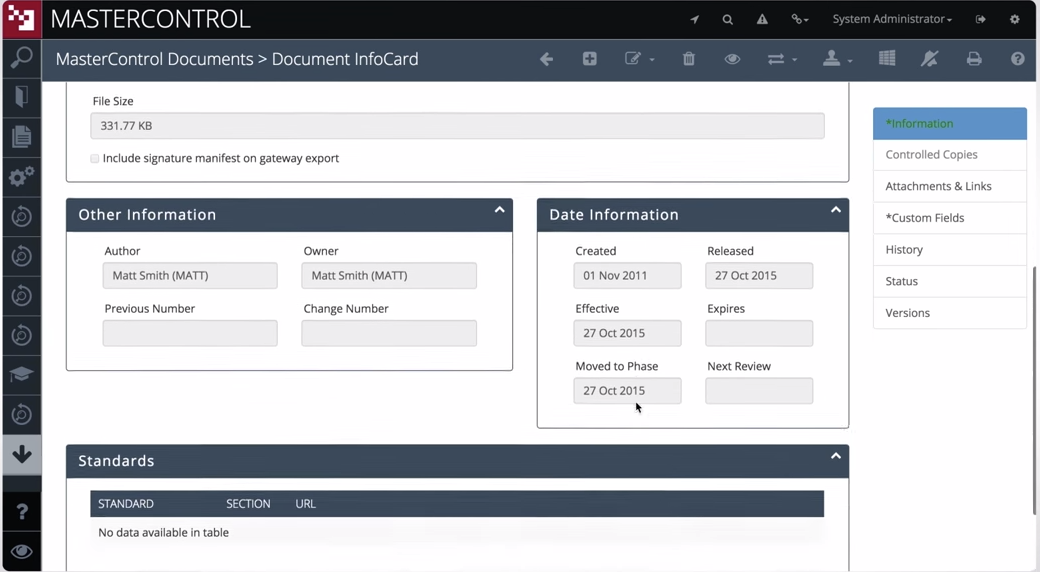
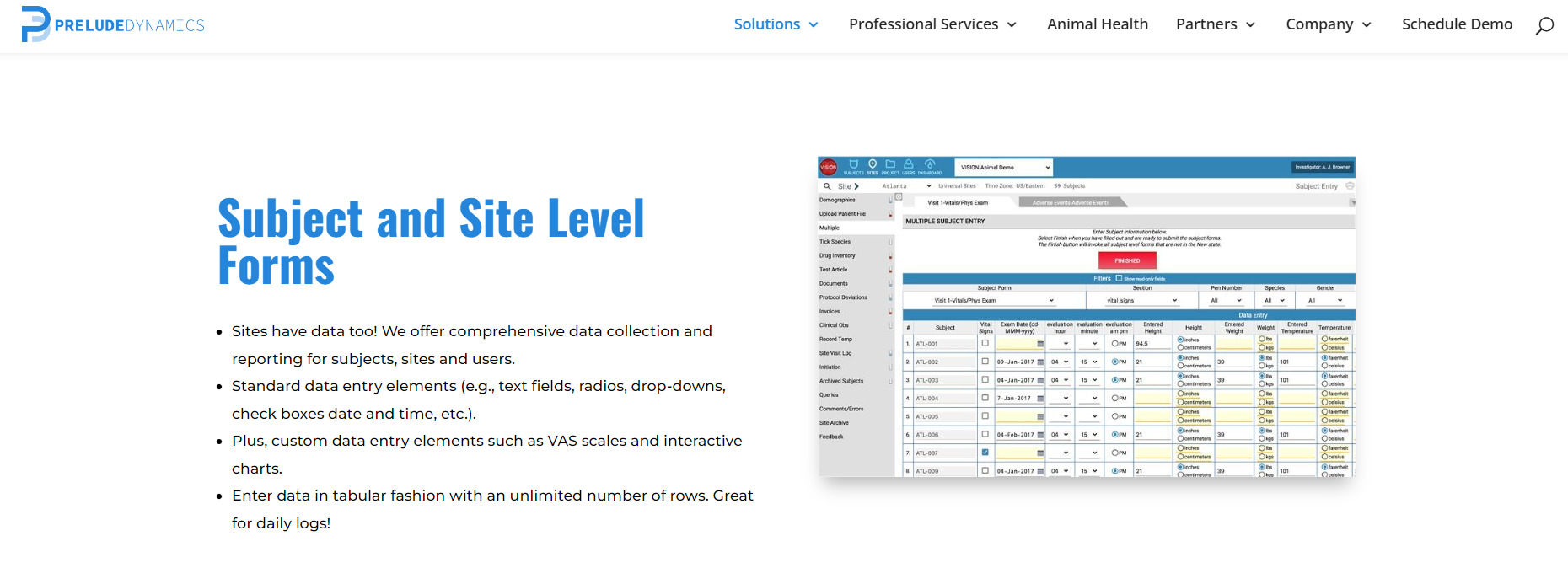
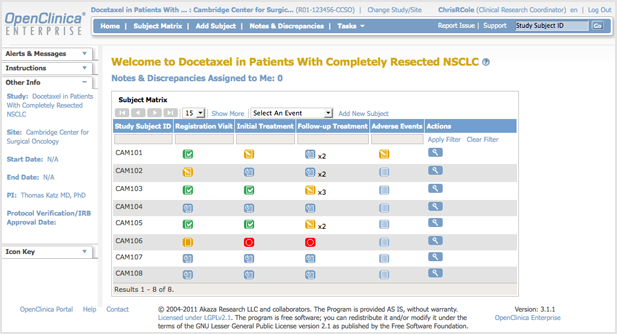
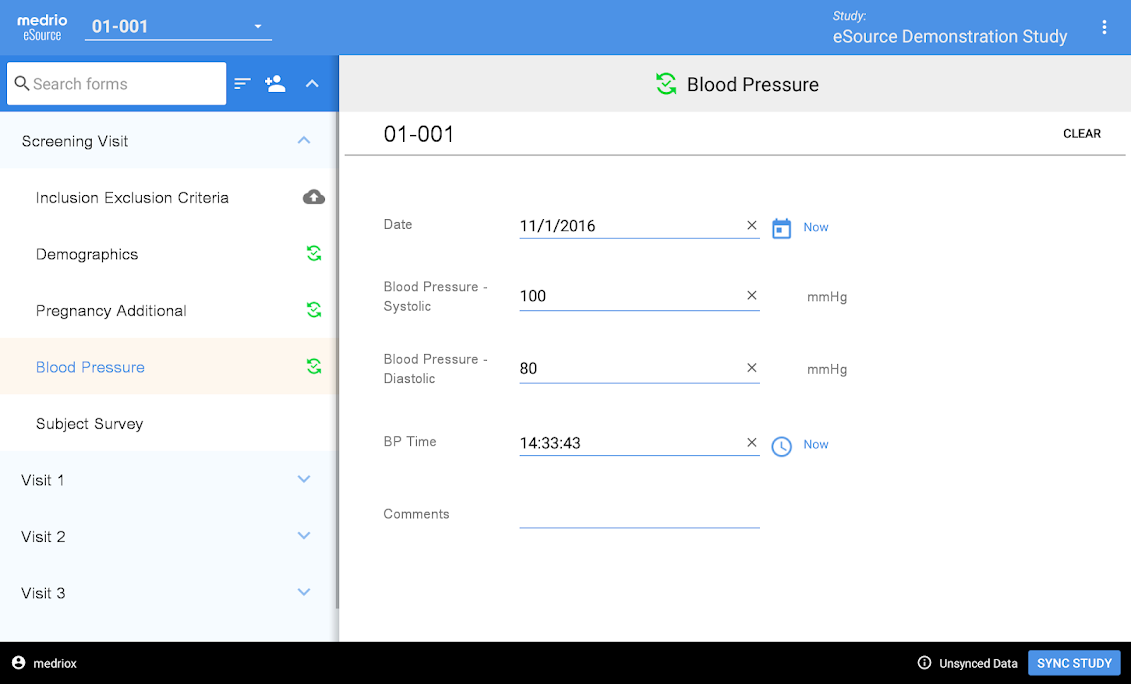
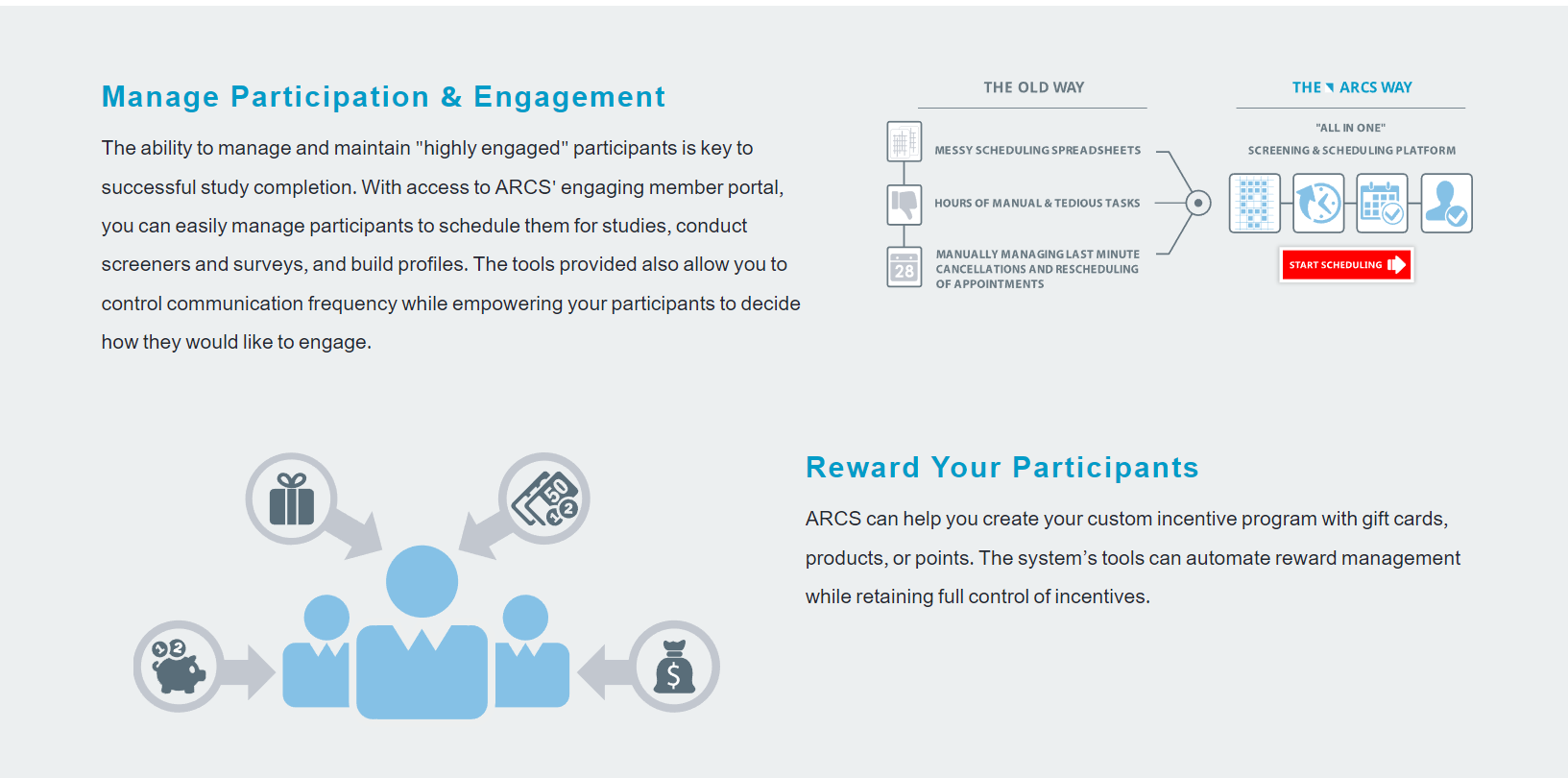
In this way, you may ensure the openness of your studies or projects and so meet applicable standards. In addition, your research process will be simplified by using LifeSphere CTMS. Documents, patient visits, and site monitoring are just some of the tasks that can be streamlined with this solution's help. The workflow-driven review and approval procedure makes it possible to further streamline all clinical trials management activities.
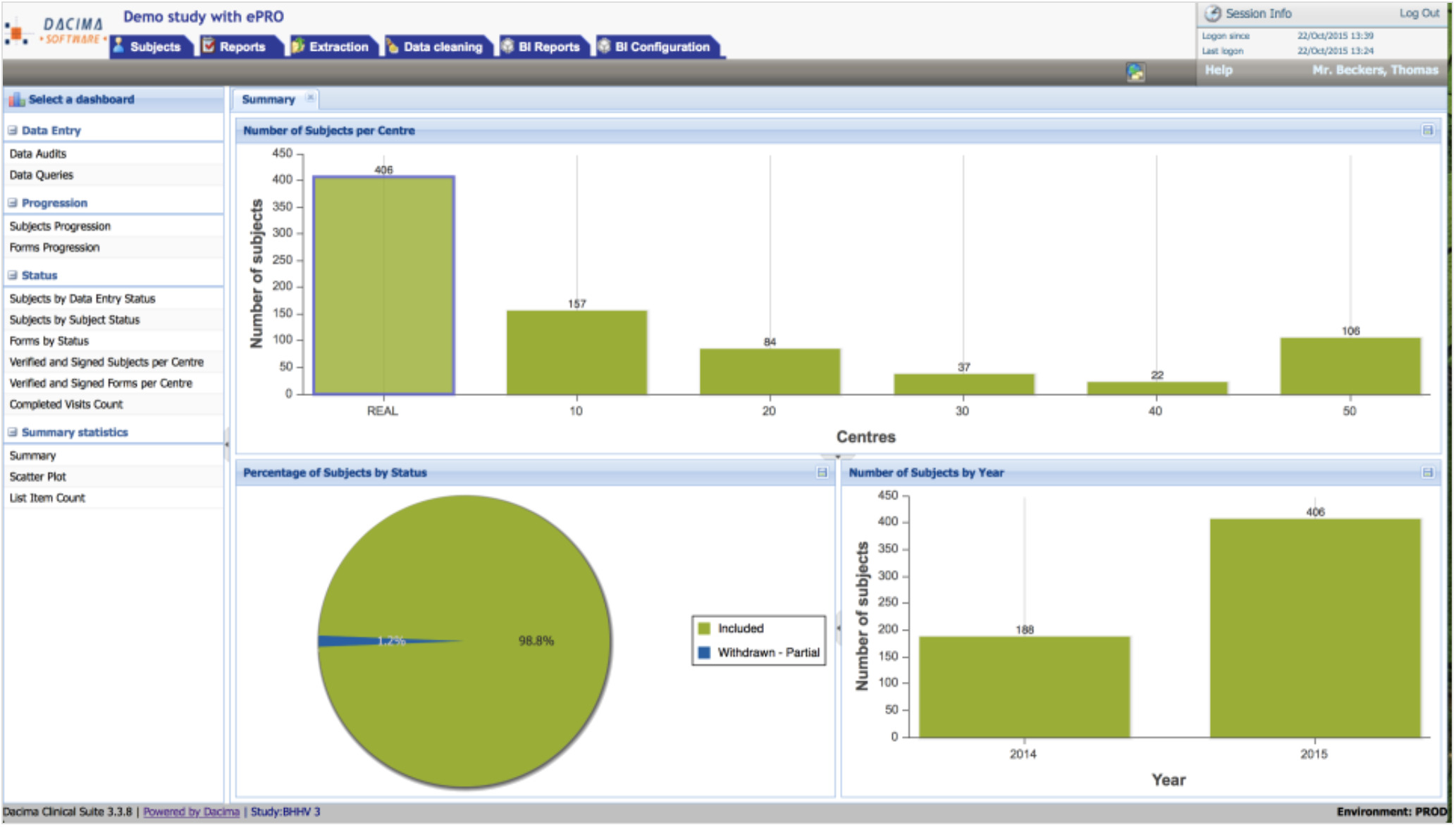
There are a bunch of decent tools out there that offer the same array of services as LifeSphere CTMS. And it can sure get confusing to choose the best from the lot. Luckily, we've got you covered with our curated lists of alternative tools to suit your unique work needs, complete with features and pricing.